On the MCAT test day, you’ll have a number of tools at your disposal that can help you identify the correct answers.
The MCAT periodic table is one of them.
However, if you aren't familiar with the table and don’t know how to use it, you won’t be able to take advantage of it on the test day.
I have helped numerous students get ready for the MCAT. Today, I’ll use my experience with MCAT preparation to talk about everything you should know relating to the MCAT periodic table.
Do You Get a Periodic Table on the MCAT?
- MCAT candidates may be asked to apply periodic table knowledge to solve questions.
- You will be provided with a periodic table that is divided into groups and trends.
- There are ten groups, encompassing metals and nonmetals.
- There are four trends that show element aspects.
Atoms and the Periodic Table

Atoms are the smallest particles of a chemical element and their defining structure [1].
They are divided into nuclei, protons, and electrons.
Atoms are the smallest units of matter which can hold a unique identity.
Together with the periodic table, atoms are the basis of a lot of general chemistry and organic chemistry questions on the MCAT.
The Periodic Table
I’ve mentioned how you’ll be given a periodic table on the MCAT [2].
You may be asked to use the periodic table and apply the knowledge of periodic trends to establish and compare element properties.
To be able to do this, you should know periodic table groups and trends.
A periodic table was devised by a Russian chemist, Dmitri Mendeleev, in 1896.
The elements in the table are divided into:
- Columns — groups
- Rows — periods
All the elements have certain properties. The properties show an element's physical state, i.e., gas, solids, or liquids, and chemical reactivity.
The periodic table lists each element’s atomic number and its relative atomic mass.
The elements are organized in a way that shows their number and electron pattern.
This is useful for predicting the element reactivity — what other elements it is likely to bond with.
Periodic Table Groups

Vertically, all 18 columns are called groups or families, and horizontally they’re called periods.
Metals are on the left, and nonmetals are on the right.
It’s important to know the placement of metals and nonmetals for boiling point questions.
If you get a molecule on the right side, you’ll know it’s a gas at room temperature, so its boiling point will be low.
Note: Many elements in a column will look and behave similarly because they have the same number of electrons in the outermost shell.
Recommended Articles:
These are periodic table groups, starting with the first one and moving forward:
- Alkali Metals — These are very reactive and can explode when in contact with water. Alkali metals have just one valence electron. *Hydrogen is also placed here, but it is considered nonmetal.
- Alkaline Earth Metals — These have two valence electrons and are highly reactive.
- Lanthanides — These are metals from 57 to 71. They are a silvery-white color.
- Actinides — These are elements 89 to 103. They are all radioactive, and together with lanthanides, form inner transition metals.
- Transition Metals — These are group 3 to group 12. These are typical metal representatives. They are hard and shiny.
- Post-Transition Metals — These are softer than transition metals and have poor conductivity. If you see a bold staircase on the periodic table, you’ve found post-transition metals.
- Metalloids — These are mostly semiconductors and include boron, silicon, germanium, arsenic, tellurium, and polonium.
- Nonmetals — All elements to the upper right of the staircase are nonmetals.
- Halogens — These are found in group 17. They are fairly reactive, and together with alkali, can create salt, such as one mostly found in kitchens.
- Noble Gases — These are nonreactive and have a full shell of valence electrons.
Periodic Trends
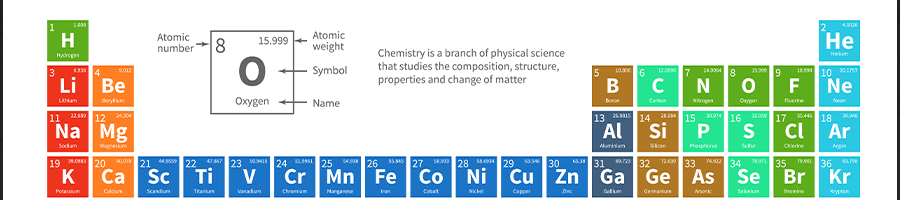
The periodic table has several trends. These are specific patterns that show aspects of an element.
“Periodic trends are very important on the MCAT. They can save you a lot of time, and get you a lot of points.” -Mcatforme YouTube Channel
- Electronegativity — This is the atom's ability to attract and bind with electrons. The scale used for calculating electronegativity is the Pauling scale.
- Ionization Energy — This is the energy needed to remove an electron from the valence shell of an atom.
- Electron Affinity — This is the ability of an atom to accept an electron.
- Atomic Radius — This is half of the distance between the two atomic nuclei. Atomic radius size decreases upwards and to the right of the periodic table.
Related Articles:
What You Should Know About the Periodic Table MCAT
I’ve covered everything you should know about the periodic table for the MCAT.
Start with the groups, and get familiar with all 18 columns. Then move onto the trends, and study how they show element patterns.
You may be asked to do numerous calculations for periodic trends, so make sure to go through as many practice tests as possible.
Finally, save this information, so you can come back to it when needed.
References:
- https://www.livescience.com/37206-atom-definition.html
- https://students-residents.aamc.org/media/8721/download
About the author

Add Comment